Oncological conditions during pregnancy are diagnosed much more frequently in recent years due to advanced diagnostic methods. With the increase of the rate of diagnosis and specific oncological treatment, there were registered changes in the obstetrical behavior, but certain side effects were also observed on the fetus and the newborn. Because in many cases it is necessary to make a decision regarding the termination of the pregnancy taking into account the maternal benefit, the involvement of future parents in the conduct during pregnancy is crucial, the obstetrician together with the oncologist and parents establishing the optimal diagnosis and the oncological treatment. Regarding the medical decisions for the pregnancies of oncological patients, the safety of the fetus, the risk of metastasis and the viability of the fetus must be taken into account. The imaging diagnostic methods can have teratogenic effects on the fetus, especially in the first trimester of pregnancy, and the oncological treatment can have an impact on fetal growth, the neurological and cardiovascular system, and it is known that cytostatics cross the placenta in large quantities. The evolution of fetuses and newborns is generally favorable in the short term, but in the long term there are not enough studies to draw a conclusion. Unfortunately, there are situations in which fetal and placental metastases are encountered and these will negatively influence the viability of the fetus. The oncological pathology during pregnancy remains a challenge, due to the side effects of diagnostic methods and treatment on the pregnant woman, on the fetus and later on the newborn.
Impactul fetal şi neonatal al metodelor de diagnostic şi al chimioterapiei în sarcină la gravidele cu afecţiuni oncologice
Fetal and neonatal impact of diagnostic methods and chemotherapy in pregnancy in women with oncological diseases
First published: 17 noiembrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.34.4.2021.5701
Abstract
Rezumat
Afecţiunile oncologice din timpul sarcinii sunt diagnosticate mult mai frecvent în ultimii ani datorită metodelor de diagnostic avansate. Odată cu creşterea ratei de diagnosticare şi tratament specific oncologic, au apărut modificări în conduita obstetricală, dar au fost observate şi anumite efecte secundare asupra fătului şi nou-născutului. Deoarece în multe cazuri se impune luarea unei decizii în ceea ce priveşte întreruperea sarcinii, ţinând cont de beneficiul matern, implicarea viitorilor părinţi în ceea ce priveşte conduita din timpul sarcinii este crucială, medicul obstetrician alături de medicul oncolog şi de părinţi stabilind schema optimă de diagnostic şi tratament oncologic. În ceea ce priveşte deciziile medicale pentru sarcinile pacientelor oncologice, trebuie ţinut cont de siguranţa fătului, de riscul de metastazare şi de viabilitatea fătului. Metodele de diagnostic imagistic pot avea efecte teratogene asupra fătului, mai ales în primul trimestru de sarcină, iar tratamentul oncologic poate avea impact asupra creşterii fătului, dar şi asupra sistemului neurologic şi cardiovascular, fiind cunoscut faptul că citostaticele traversează placenta în cantitate mare. Evoluţia feţilor şi nou-născuţilor este favorabilă în general pe termen scurt, însă pe termen lung nu există suficiente studii pentru a trage o concluzie. Din nefericire, există situaţii în care se întâlnesc metastaze fetale şi placentare care vor influenţa negativ viabilitatea fătului. Patologia oncologică în timpul sarcinii rămâne o provocare, din cauza efectelor secundare ale metodelor de diagnostic şi ale tratamentului asupra femeii gravide, fătului şi ulterior asupra nou-născutului.
Introduction
The incidence of cancer during pregnancy has increased in recent decades, with doctors facing a complex process of making cancer-obstetric decisions.
Because the effects on the fetus and newborn, in the short term, after cancer treatment, are soothing, more women receive treatment during pregnancy. The prenatal treatment should follow the standard treatment as much as possible to optimize the maternal prognosis, always taking into account the fetal well-being. To ensure the optimal treatment for both mother and fetus, a multidisciplinary team of specialists should be involved in the decision-making.
In addition to oncology treatment, an obstetric and perinatal management plan, agreed with future parents, is crucial. The results of noninvasive prenatal testing are inconclusive in women with cancer and alternatives should be used for the prenatal screening of the disease. Chemotherapy in pregnant women requires repeated ultrasound examinations to monitor the fetal growth and the appearance of the uterus. After birth, a neonatal evaluation allows the clinical identification of the adverse effects caused by cancer or its treatments. In addition, placental histological examination aims to assess the fetal risk of metastases.
In the last 20 years, the clinical management of pregnant women with cancer has evolved, with a higher number of patients receiving treatment during pregnancy, with rarer cases of abortion and fewer iatrogenic-induced premature births(1). In addition, cancer may be accompanied by maternal stress, inflammatory reactions, radiation exposure, anesthetics and other medications, which may influence the fetal development. However, data on the short- and long-term impact on fetal development are still limited(2).
Types of cancer during pregnancy
The incidence of oncological pathology in pregnancy for the mother is about 1:1000(3). In a recent Italian population study, breast cancer was the most common cancer in pregnancy (32%, n=479) and the risk of developing cancer during pregnancy increased significantly with age, from 60 in 100,000 women under the age of 30 to 265 in 100,000 women over the age of 40(4). Melanoma, breast cancer and thyroid cancer were more common in the Australian population than reported in other countries, and the incidence of cervical and ovarian cancer was lower(5). In an international cohort of 1170 women diagnosed with cancer during pregnancy, the most common types of invasive cancer were breast cancer (39%, n=462), followed by cervical cancer (13%, n=147), lymphoma (10%, n=113), ovarian cancer (7%, n=88) and leukemia (6%, n=68)(1). A study based on the Swedish population found that the most common type of cancer during pregnancy was melanoma (25%, n=232), followed by breast cancer (15%, n=139), cervical cancer (15%, n=139) and ovarian cancer (6%, n=54)(6).
Diagnostic methods
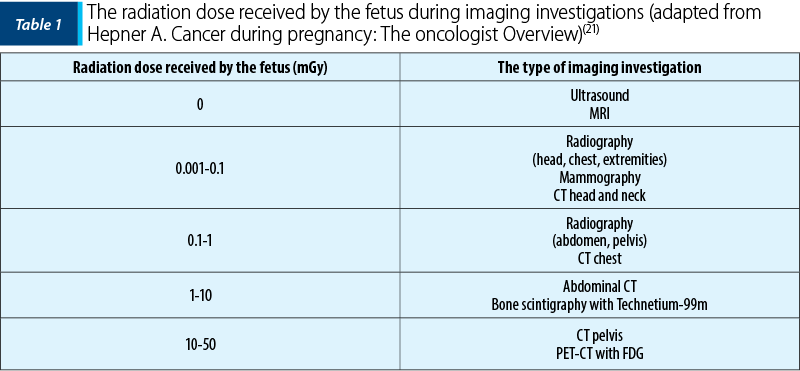
Assessing breast symptoms during pregnancy and the postpartum period can be difficult due to hormone-induced changes in breast tissue that can lead to increased firmness and nodularity. In addition, the symptoms of postpartum lactation mastitis mimic locally advanced or inflammatory breast cancer. Most forms of pregnancy-associated breast cancer (PABC) are diagnosed after a palpable mass is detected. Thickening of the skin and redness of the skin may also be present(7,8). Completing the diagnostic process with medical imaging to determine the extent of the disease is important in making treatment decisions. In a non-pregnant patient, breast imaging may include ultrasound, mammography and nuclear magnetic resonance imaging (MRI) of the breasts. Ultrasound helps to differentiate cystic formations from solid ones, and mammography can show calcifications that may not be visible on ultrasound(9). Ultrasound is widely used during pregnancy due to the safety of this imaging method(10). Mammography with abdominal protection (shielding) exposes the fetus to minimal doses (0.001-0.01 mGy with two views), well below the minimum threshold of 200 mGy for side effects during organogenesis (up to 10 weeks of gestation)(11).
Imaging used during pregnancy to diagnose and stage cancer can create conflicts between maternal benefit and fetal risk. Therefore, the following aspects must be taken into account when choosing the appropriate imaging evaluation technique: fetal safety, risk of metastasis, viability of the fetus.
Breast MRI with improved contrast may be a useful diagnostic tool in non-PABC, and the safety of gadolinium during pregnancy is controversial. Free gadolinium is considered toxic and is therefore administered to humans only in chelated form. It crosses the placenta and remains in the amniotic fluid, is swallowed by the fetus and reenters the fetal circulation. Moreover, the positioning required to perform breast MRI can lead to prolonged pressure on the pregnant uterus, disrupting the uterine blood flow (uteroplacental hypoperfusion). Breastfeeding women are encouraged to use the milking pump or breastfeed immediately before MRI to improve contrast. Gadolinium is excreted in breast milk at a rate of 0.0004% of the breast dose, and therefore the guidelines from the American College of Radiology do not indicate the discontinuation of breastfeeding after contrast-enhanced MRI(12). In some cases of advanced PABC where metastases are suspected, appropriate radiological preparation may be required prior to birth to guide the therapeutic decisions. Because the lungs, bone and liver are the most common metastatic sites for breast cancer, a pregnant patient may have a chest X-ray with abdominal shielding, liver ultrasound and non-contrast MRI in supine position instead of bone X-rays for metastasis assessment. The fetal PET/CT dose was found to be 10-50 mGy and is therefore usually delayed until the postpartum period.
In non-pregnant patients, computed tomography fluoride-18-flurodeoxyglucose emission tomography (18FDG-PET/CT) is a valuable tool for the detection and staging of various cancers (especially hematologic cancer and staging advanced breast cancer). However, 18FDG-PET/CT should be avoided during pregnancy due to the high dose of radiation exposure to the fetus (up to 50 mGy)(13). Diffusion-weighted MRI (DWI-MRI) for the whole body has been shown to be feasible in detecting primary lesions and nodal along with distant metastases in women diagnosed with cancer during pregnancy. Furthermore, it can be used as a screening tool for the underlying malignancy in a noninvasive abnormal prenatal test(14). As no adverse effects on the fetus have been identified, this non-ionizing imaging technique can be used safely. If indicated, pineapple juice can be used as an oral negative contrast agent for imaging optimization of the abdomen without affecting the fetus(15).
The protocols for one day treatment of the sentinel lymph node are considered safe during pregnancy(16). Radioisotopes may be used for these procedures when administered in small amounts, not exceeding 5 mGy fetal exposure(17). The lowest possible dose of technetium can be injected topically two hours before the procedure. In such cases, approximately 90% of technetium will be collected in the sentinel node, resulting in low systemic exposure and minimal fetal risk. Indocyanine green, known for accurate sentinel node detection and very limited placental transfer, is widely used in pregnant patients(18,19). The use of methylene blue dye for sentinel node detection should be avoided as there is a low risk of anaphylactic reaction (0.1%)(20).
Cervical cancer is best diagnosed by cytology in early pregnancy, with the following symptoms: abnormal bleeding, vaginal discharge and abdominopelvic pain(22). Cervical cancer during pregnancy is generally stage I at the time of diagnosis, with pregnant women being three times more likely to have stage I disease than non-pregnant women(23).
The treatment of preinvasive disease (CIN 1 to CIN 3) can be delayed for up to 6-8 weeks after birth. Thus, it is recommended to perform a colposcopy in each pregnancy trimester to assess the size of the lesion and the progression of the disease. Colposcopy can be a challenge during pregnancy, secondary to increased vascularity and increased genital edema(24).
Stage IA1 can be managed by conization, performed especially between 12 and 20 weeks of pregnancy(25). The term “coin biopsy” is sometimes used during pregnancy to indicate that the incision should not be deep enough to cause damage to the fetal membranes. The prophylactic cerclage can be an option both for the prevention of premature labor and for the management of operative bleeding. In stages IA2-IB1 (less than 2 cm), conization or simple trachelectomy can be performed because the parametric extension is observed in less than 1% of women. Stage IB1 tumors larger than 2 cm can be treated with neoadjuvant chemotherapy (NACT) with or without pelvic lymphadenectomy.
Chemotherapy is relatively safe in the second trimester, although there is a higher risk of premature labor, premature rupture of membranes, and restriction of fetal growth. NACT stabilizes the tumor until fetal maturity is reached, so that caesarean delivery and radical hysterectomy can be performed. In women with advanced pregnancy (above 22-25 weeks), pelvic lymphadenectomy is not possible, and in those with disease from stage IA1 to stage IB1, the treatment can be delayed until fetal maturity is reached without compromising survival. NACT can be given in both advanced local cancer and in the third trimester to keep the pregnancy up to an optimal fetal prognosis (35-36 weeks). Caesarean birth followed by radical surgery or permanent chemotherapy/radiotherapy showed a good obstetrical and oncological result(26). Caesarean delivery is the preferred choice for fetal extraction in the presence of large tumors. Vaginal birth comes with the possibility of major bleeding and the implantation of metastases in vaginal lacerations or episiotomy scars, being contraindicated. In locally advanced tumors, birth by segmental-transverse caesarean section should be avoided due to the risk of sectioning or rupture of tumor tissue. A classic incision will reduce blood loss and avoid intercepting large tumor vessels. Where it is not desired to preserve the pregnancy or in advanced stages, it is recommended to terminate the pregnancy and institute the same treatment as in women who are not pregnant. During early pregnancy (less than 12 weeks), miscarriage occurs after pelvic irradiation. In the second trimester, hysterectomy followed by radiochemotherapy is preferred because the obstetric complications are fewer.
Oncological treatment
The oncological treatment may have acute and/or chronic side effects on the fetus, including neurotoxicity and cardiotoxicity, as cytostatics may cross the placenta in varying amounts(27,28). In general, cancer treatment during pregnancy should adhere to medications used for non-pregnant patients as much as possible in order to preserve the maternal prognosis. In addition to surgery, systemic treatment, if compatible with pregnancy, plays an important role in prenatal cancer treatment. Abortion may be considered in the case of aggressive or advanced cancer in early pregnancy. Premature induction of birth to start cancer treatment should be avoided where possible due to the long-term morbidity of preterm infants.
The change in cancer treatment and the impact during pregnancy were reflected in an international cohort study(4). For every five years of the study (1996-2016), the rate of oncological treatment use increased by 10% and the rate of use of chemotherapy increased by 31%. The percentage of live births has increased and premature births have decreased. Maternal survival was similar to that of non-pregnant women treated for cancer and encouraging results were observed in fetuses, neonates and in early childhood. As such, the oncological treatment is possible during pregnancy, often without compromising maternal or fetal safety.
The transplacental transfer of chemotherapeutic drugs takes place by passive diffusion and is therefore based on drug-specific molecular size, lipid solubility, protein binding and ionization. Because most chemotherapeutic drugs have a low molecular weight and are unloaded and unbound, they can easily cross the human placenta. In baboons, the switch from fetus to mother ranged from zero for docetaxel to a maximum concentration ratio of 0.58 for carboplatin(27,28).
Chemotherapy should be avoided during the first trimester of pregnancy to avoid interference with organogenesis. After 12-14 weeks of gestation, the administration of most cytotoxic drugs is feasible and considered relatively safe(29). The standard regimens and doses of chemotherapy using actual maternal weight during pregnancy are preferred. After 35 weeks of gestation, chemotherapy is usually discouraged to allow a certain window in the administration schedule for maternal and fetal bone marrow recovery between the last cycle of chemotherapy and birth. The latter should be ideally planned, allowing the resumption of postpartum chemotherapy in a timely manner if indicated.
Imatinib, a tyrosine kinase inhibitor approved for the treatment of Philadelphia-positive chronic myeloid leukemia, crosses the placenta and should not be given in the first trimester. It has been shown to cause malformations when given in the first trimester in pregnant women, but it appears safe in the second and third trimesters(30).
Thus, antivascular and other antiangiogenic endothelial growth factors are contraindicated during pregnancy. Targeted HER-2 therapy (trastuzumab), commonly used to treat HER-2 positive breast cancer, is associated with severe oligo-/anhydramnios and with subsequent neonatal respiratory failure due to pulmonary hypoplasia when administered in the second or third trimester, probably due to blockage of the epidermal growth factor receptor 2 secreted in the fetal kidney(31).
The supportive medication as part of systemic treatment is considered safe for a number of medications. Antiemetics may be administered during pregnancy, including metoclopramide and serotonin receptor antagonists(32), but the safety has not been determined for neurokinin-1 inhibitors(33).
There are ongoing debates about the use of growth factors, such as granulocyte colony stimulating factor and erythropoietin, although the former has been shown to be safe during pregnancy, allowing for high-dose treatment programs(34).
Fetal and neonatal results
The short-term outcomes of fetuses and newborns exposed to intrauterine chemotherapy are generally satisfactory; however, the long-term results (>6 years) are unknown and the use of more hormonal and targeted therapies should be discouraged until more information on fetal safety is available.
As shown above, there is a risk of malformations for the fetus during the first trimester of pregnancy under the influence of certain therapies and methods of diagnostic imaging.
A recent Italian monocentric study found a trend toward fewer elective caesarean sections in the last decade for patients diagnosed with cancer during pregnancy(35). This has been accompanied by an increasing percentage of labor inductions, mainly due to the need to start or continue chemotherapy(35). A higher incidence of neonatal respiratory distress has also been observed, largely due to iatrogenic-induced prematurity. The outcome in terms of cognitive and cardiac development in the first six years after exposure to chemotherapy is not significantly affected compared to children in the general population(36,37). Certain precautions are necessary because chemotherapy during pregnancy is associated with impaired fetal growth and when given at the beginning of pregnancy(1).
Neonatal/fetal metastases have been found mainly in patients with melanoma and also in lung cancer and leukemia. Placental metastases are located mainly in the interciliar space and to a lesser extent in the villi, probably due to the placental barrier that protects the fetus from dangerous substances in the mother’s circulation. Although, based on limited evidence, only villous involvement has been described in association with neonatal metastases. To this end, the histological examination of the placenta is crucial and recommended for the detection of microscopic placental metastases and the identification of potential fetal involvement, especially in women with melanoma or advanced cancer.
As mentioned earlier, fetal growth restriction is a well-known obstetric complication in complicated cancer pregnancies(1). Uteroplacental vascular insufficiency and the subsequent impact on placental development are the main causes of intrauterine growth restriction in the general population(38). Premature rupture of membranes and premature birth have been observed in mothers with oncological pathology and chemotherapy during pregnancy(26).
Bone marrow toxicity may occur in newborns prenatally exposed to chemotherapy and fetal anemia secondary to cancer or treatment can be detected by Doppler ultrasound examination(39). The combination of altered general maternal health, pancytopenia, systemic disease, oncological stress and cytotoxic drugs may endanger the fetus and placental function, leading to an increased risk of intrauterine fetal death, especially in patients with acute fetal leukemia. Intrauterine death in leukemia is more common compared to other cancers.
Breastfeeding
Breastfeeding is discouraged when the systemic treatment should be continued after birth. An interval of at least three weeks between the last treatment and breastfeeding is recommended to prevent any treatment-induced neonatal effect from most non-platinum chemotherapeutic agents.
Women receiving chemotherapy or treatment with tamoxifen should not breastfeed.
There is no evidence that breastfeeding increases the risk of developing breast cancer or for developing secondary breast cancer, nor that it poses a risk to the child’s health. Women previously treated for breast cancer who show no signs of a residual tumor should be encouraged to breastfeed.
Conclusions
Overall, obstetric outcomes have improved in recent decades, with fewer miscarriages and fewer preterm births. Changes in obstetric management have been driven by a better understanding of cancer during pregnancy and by a general tendency to reduce the effects of toxicity on the fetus and newborn due to chemotherapy. Furthermore, pregnant women and their partners should be supported and encouraged to be actively involved in the decision-making on cancer treatment and perinatal decisions. In addition, postpartum guidance on breastfeeding, physiological and psychological well-being is often highly desirable. Oncological pathology during pregnancy remains a challenge, due to the side effects of diagnostic methods and treatment on the pregnant woman, the fetus and later on the newborn.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
Vandenbroucke T, Verheecke M, Fumagalli M, Lok C, Amant F. Effects of cancer treatment during pregnancy on fetal and child development. Lancet Child Adolesc Health. 2017;1(4):302-10.
Salani R, Billingsley CC, Crafton SM. Cancer and pregnancy: an overview for obstetricians and gynecologists. Am J Obstet Gynecol. 2014;211(1):7-14.
Parazzini F, Franchi M, Tavani A, Negri E, Peccatori FA. Frequency of pregnancy related cancer: a population-based linkage study in Lombardy, Italy. Int J Gynecol Cancer. 2017;27(3):613-9.
Lee YY, Roberts CL, Dobbins T, Stavrou E, Black K, Morris J, Young J. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994-2008: a population-based linkage study. BJOG. 2012;119(13):1572-82.
Andersson TM, Johansson AL, Fredriksson I, Lambe M. Cancer during pregnancy and the postpartum period: a population-based study. Cancer. 2015;121(12):2072-7.
Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Burke B, Sutcliffe SB, Billingsley G. Maternal and fetal outcome after breast cancer in pregnancy. Am J Obstet Gynecol. 1992;166(3):781-7.
Al-Amri AM. Clinical presentation and causes of the delayed diagnosis of breast cancer in patients with pregnancy associated breast cancer. J Family Community Med. 2015;22(2):96-100.
Vashi R, Hooley R, Butler R, Geisel J, Philpotts L. Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR Am J Roentgenol. 2013;200(2):321-8.
Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2015;2015(7):CD007058.
Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol. 2017;130(4):e210-e216.
McCollough CH, Schueler BA, Atwell TD, Braun NN, Regner DM, Brown DL, LeRoy AJ. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27(4):909-17;discussion 917-8.
American College of Radiology. ACR-SPR practice parameter for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation. Resolution 39, 2018. Available at: https://www.acr. org/-/ media/ ACR/ Files/ Practice- Parameters/ Pregnant- Pts. Pdf
Han SN, Amant F, Michielsen K, De Keyzer F, Fieuws S, Van Calsteren K, Dresen RC, Gziri MM, Vandecaveye V. Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: a pilot study. Eur Radiol. 2018;28(5):1862-74.
Frisch A, Walter TC, Hamm B, Denecke T. Efficacy of oral contrast agents for upper gastrointestinal signal suppression in MRCP: A systematic review of the literature. Acta Radiol Open. 2017;6(9):2058460117727315.
Han SN, Amant F, Cardonick EH, Loibl S, Peccatori FA, Gheysens O, Sangalli CA, Nekljudova V, Steffensen KD, Mhallem Gziri M, Schröder CP, Lok CAR, Verest A, Neven P, Smeets A, Pruneri G, Cremonesi M, Gentilini O; International Network on Cancer, Infertility and Pregnancy. Axillary staging for breast cancer during pregnancy: feasibility and safety of sentinel lymph node biopsy. Breast Cancer Res Treat. 2018;168(2):551-7.
Adelstein SJ. Administered radionuclides in pregnancy. Teratology. 1999;59(4):236-9.
Rychlik A, Marin S, De Santiago J, Zapardiel I. Utility of laparoscopic Indocyanine green-guided sentinel node biopsy in open cervical cancer surgery. Int J Gynecol Cancer. 2016;26(7):1288-9.
Rubinchik-Stern M, Shmuel M, Bar J, Eyal S, Kovo M. Maternal-fetal transfer of indocyanine green across the perfused human placenta. Reprod Toxicol. 2016;62:100-5.
Bézu C, Coutant C, Salengro A, Daraï E, Rouzier R, Uzan S. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol. 2011;20(1):e55-9.
Hepner A, Negrini D, Hase EA, Exman P, Testa L, Trinconi AF, Filassi JR, Francisco RPV, Zugaib M, O’Connor TL, Martin MG. Cancer during pregnancy: the oncologist overview. World J Oncol. 2019;10(1):28-34.
Sekine M, Kobayashi Y, Tabata T, Sudo T, Nishimura R, Matsuo K, Grubbs BH, Enomoto T, Ikeda T. Malignancy during pregnancy in Japan: an exceptional opportunity for early diagnosis. BMC Pregnancy Childbirth. 2018;18(1):50.
Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012;379(9815):558-69.
Hunter MI, Monk BJ, Tewari KS. Cervical neoplasia in pregnancy. Part 1: screening and management of preinvasive disease. Am J Obstet Gynecol. 2008;199(1):3-9.
Amant F, Halaska MJ, Fumagalli M, Dahl Steffensen K, Lok C, Van Calsteren K, Han SN, Mir O, Fruscio R, Uzan C, Maxwell C, Dekrem J, Strauven G, Mhallem Gziri M, Kesic V, Berveiller P, van den Heuvel F, Ottevanger PB, Vergote I, Lishner M, Morice P, Nulman I; ESGO task force ‘Cancer in Pregnancy’. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer. 2014;24(3):394-403.
Ricci C, Scambia G, De Vincenzo R. Locally Advanced Cervical Cancer in Pregnancy: Overcoming the Challenge. A Case Series and Review of the Literature. Int J Gynecol Cancer. 2016;26(8):1490-6.
Van Calsteren K, Verbesselt R, Beijnen J, Devlieger R, De Catte L, Chai DC, Van Bree R, Heyns L, de Hoon J, Amant F. Transplacental transfer of anthracyclines, vinblastine, and 4-hydroxy-cyclophosphamide in a baboon model. Gynecol Oncol. 2010;119(3):594-600.
Van Calsteren K, Verbesselt R, Devlieger R, De Catte L, Chai DC, Van Bree R, Heyns L, Beijnen J, Demarsin S, de Bruijn E, de Hoon J, Amant F. Transplacental transfer of paclitaxel, docetaxel, carboplatin, and trastuzumab in a baboon model. Int J Gynecol Cancer. 2010;20(9):1456-64.
Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283-91.
Lambertini M, Peccatori FA, Azim HA Jr. Targeted agents for cancer treatment during pregnancy. Cancer Treat Rev. 2015;41(4):301-9.
Azim HA Jr, Metzger-Filho O, de Azambuja E, Loibl S, Focant F, Gresko E, Arfi M, Piccart-Gebhart M. Pregnancy occurring during or following adjuvant trastuzumab in patients enrolled in the HERA trial (BIG 01-01). Breast Cancer Res Treat. 2012;133(1):387-91.
Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368(9):814-23.
Briggs G, Freeman R, Yaffe S. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. Wolters Kluwer Health/Lippincott Williams&Wilkins, 2011.
Cardonick E, Irfan F, Torres N. The use of Neupogen (filgrastim) or Neulasta (pegfilgrastim) during pregnancy when chemotherapy is indicated for maternal cancer treatment. J Cancer Ther. 2012;03:157–61.
Masturzo B, Parpinel G, Macchi C, De Ruvo D, Paracchini S, Baima Poma C, Danna P, Pagliardini G, Zola P. Impact of cancer in the management of delivery: 10 years of variations. J Matern Fetal Neonatal Med. 2020;33(12):2006-11.
Amant F, Vandenbroucke T, Verheecke M, Fumagalli M, Halaska MJ, Boere I, Han S, Gziri MM, Peccatori F, Rob L, Lok C, Witteveen P, Voigt JU, Naulaers G, Vallaeys L, Van den Heuvel F, Lagae L, Mertens L, Claes L, Van Calsteren K; International Network on Cancer, Infertility, and Pregnancy (INCIP). Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373(19):1824-34.
Vandenbroucke T, Verheecke M, van Gerwen M, Van Calsteren K, Halaska MJ, Fumagalli M, Fruscio R, Gandhi A, Veening M, Lagae L, Ottevanger PB, Voigt JU, de Haan J, Gziri MM, Maggen C, Mertens L, Naulaers G, Claes L, Amant F; International Network on Cancer, Infertility and Pregnancy (INCIP). Child development at 6 years after maternal cancer diagnosis and treatment during pregnancy. Eur J Cancer. 2020;138:57-67.
Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S745-S761.
Mari G, Adrignolo A, Abuhamad AZ, Pirhonen J, Jones DC, Ludomirsky A, Copel JA. Diagnosis of fetal anemia with Doppler ultrasound in the pregnancy complicated by maternal blood group immunization. Ultrasound Obstet Gynecol. 1995;5(6):400-5.
Articole din ediţiile anterioare
Modificările statusului periodontal în sarcină – review şi prezentare a unui caz clinic
Sarcina este o perioadă caracterizată de schimbări fiziologice legate de o vulnerabilitate ridicată a sănătăţii orale. Variaţia prevalenţei modific...
Corelaţii anatomoclinice şi ecografice în hipertensiunea arterială indusă de sarcină
Hipertensiunea arterială indusă de sarcină este diagnosticată prin creşterea tensiunii arteriale sistolice peste 140 mmHg şi a celei diastolice...
Depresia perinatală
Depresia postpartum reprezintă un subiect important în obstetrică, afectând 13% dintre femei în timpul sarcinii sau în primul an post-partum, du...
Rolul infecţiilor orale în naşterea prematură
Prematuritatea şi greutatea scăzută la naştere sunt unele din complicaţiile frecvente ale sarcinilor. Infecţiile orale, în special bolile parodonta...