Acute liver failure (ALF) is a devastating syndrome in neonates, infants or children, with a very high mortality in the absence of urgent liver transplantation. The etiology of ALF in children differs from that of adults and is represented mainly by toxics, metabolic disorders, infections, autoimmune hepatitis, vascular causes and malignancies. The etiology of ALF is related to the age of the child. Infectious causes and metabolic diseases are more common in neonates and infants, while in older children and teenagers, the most common causes are toxic, autoimmune hepatitis, and Wilson’s disease. It is a significant finding, given that in some situations, knowing the possible etiology can be life-saving, by the early introduction of a special regimen (in inborn errors of metabolism), the administration of an antidote (in intoxications) or using specific drugs (chelators in Wilson’s disease). The article aims to summarize the leading causes of ALF in children and the characteristics related to age.
Etiologia insuficienţei hepatice acute la copii
Etiology of acute liver failure in children
First published: 30 septembrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.63.3.2021.5483
Abstract
Rezumat
Insuficienţa hepatică acută (IHA) reprezintă o patologie cu o mortalitate foarte mare la nou-născuţi, sugari sau copii, în absenţa transplantului hepatic de urgenţă. Etiologia IHA la copii este diferită de cea de la adulţi şi este reprezentată în principal de toxice, tulburări metabolice, infecţii, autoimunitate şi, mai rar, de cauze vasculare şi tumori maligne. Etiologia IHA variază cu vârsta copilului. Cauzele infecţioase şi bolile metabolice sunt mai frecvente la nou-născuţi şi sugari, în timp ce la copiii mai mari şi la adolescenţi cele mai frecvente cauze sunt toxice, hepatita autoimună şi boala Wilson. Aceasta este o constatare extrem de importantă, având în vedere faptul că, în unele situaţii, cunoaşterea etiologiei poate salva viaţa, prin introducerea rapidă a unui regim special (aşa cum se întâmplă în erorile înnăscute de metabolism), prin administrarea unui antidot (în intoxicaţii) sau utilizând medicamente specifice (chelatori în boala Wilson). Scopul acestui articol este de a prezenta principalele cauze ale IHA la copii, precum şi caracteristicile legate de vârstă.
Introduction
Acute liver failure (ALF) in children is a rare and severe disease, which can be associated with a high a mortality in the absence of liver transplantation. In 1999, a multidisciplinary team composed of 24 researchers from the USA, Canada and Europe introduced the PALF criteria for children with ALF. According to them, ALF in children is a syndrome characterized by uncorrectable hepatic coagulopathy after parenteral administration of vitamin K, respectively an INR (International Normalized Ratio) of 1.5-1.9 in the presence of hepatic encephalopathy or INR>2 in the absence of hepatic encephalopathy without having a chronic liver disease(1). The etiology of ALF is different from that in adults and depends on age.
I. Infections
In Europe, the viral infections are currently the leading cause of ALF in children. Depending on the liver cell tropism of the infectious virus (specific or nonspecific), the viral agents that can cause ALF in children are classified into hepatotropic and nonhepatotropic agents(1,2).
I.1. Hepatotropic viruses
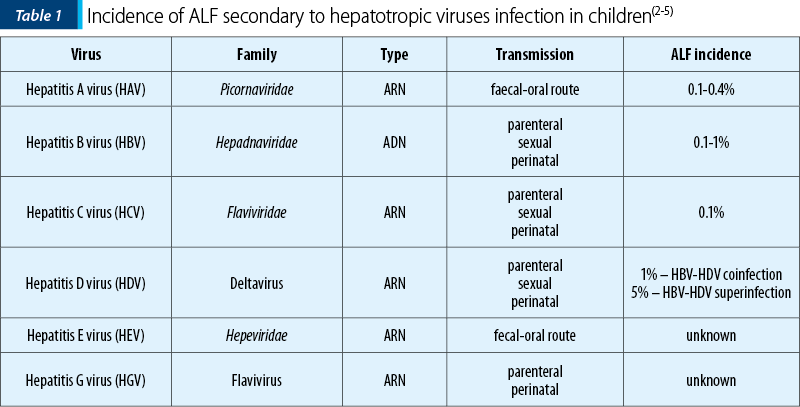
The viral etiology of ALF varies geographically, hepatitis A virus (HAV) being one of the most common etiological agents in Asia and South America, while in Africa, Europe or Asia, the infection with hepatitis B virus (HBV) is a significant public health issue. Table 1 presents the main hepatotropic viruses that can cause ALF in children(1,2).
Hepatitis A virus (HAV)
HAV is an RNA virus that belongs to the Hepatoviruses genus, family Picornaviridae. It is transmitted by the fecal-oral route and is the most common cause of hepatitis in developing countries (20-25%). The prevalence of HAV infection is increased in Asia, Southern Europe and South America, representing a major public health problem. The most common infection is mild or self-limiting, but sometimes HAV can cause severe hepatitis or ALF and other complications. The incidence of ALF cases secondary to HAV infection in children varies between 0.1% and 0.4%(6,7).
Hepatitis B virus (HBV)
HBV infection is a public health problem worldwide, highly prevalent in Europe, Asia, Africa and Latin America (5-20%). According to the World Health Organization (WHO), about a quarter of the world’s population (about 2-3 billion) has gone through HBV infection, and over 3% (250-300 million) are chronically infected with HBV. In endemic areas, the transmission of the infection is mainly perinatal (2-5%) or intrapartum (95%), while in developed countries, sexual transmission, tattooing or injecting drugs are the main ways of HBV infection. HBV infection can evolve with ALF at any age, with an incidence of 0.1-0.5% and a mortality of up to 80%. The highest risk of developing ALF is posed by children born to mothers with high viremia. They may develop ALF in the first 3-10 weeks of life, with an extremely high mortality rate, or progress to chronic infection in 90% of cases. The evolution of the infection to fulminant forms or chronicity depends on the HBV genotype and the host’s immune status. This is one of the main reasons the neonates and infants present severe forms of HBV infection(8-11).
Hepatitis C virus (HCV)
HCV infection rarely causes ALF in children, the prevalence of HCV infection in children and adolescents in Europe being below 0.2%, except for endemic areas where it can reach up to 5%. Most of the time, the transmission is maternal-fetal (60% of infected children), the risk being amplified if mothers are HIV coinfected or if they have high viremia during pregnancy. Among older children, those who receive regular blood transfusions, perform extracorporeal treatment methods, or adolescents who inject drugs are at an increased risk of infection(12,13).
Hepatitis D virus (HDV)
HDV is more common in areas where HBV is endemic, with a low socioeconomic level (Mediterranean basin, Eastern Europe, Asia or Africa). The virus can cause coinfection (the infection occurs concomitantly with HBV) or superinfection (a patient already infected with HBV becomes infected with HDV). Coinfection is more frequently associated with severe forms of the disease, sometimes fulminant (70-80%), while superinfection progresses mainly to chronicity (90% of cases)(13,14).
Hepatitis E virus (HEV)
HEV infection is a rare cause of ALF in children, although in adults it is one of the leading causes of severe liver injuries in developing countries (20-40% of cases). It is endemic mainly in tropical countries, where it causes approximately 70,000 deaths/year. HEV belongs to the family Hepeviridae, genus Hepevirus, and is transmitted by the fecal-oral route, especially by contaminated water or secondary to the consumption of contaminated meat. Not least, the virus can be transmitted through the administration of blood products from infected donors. Most of the time, the infection is self-limiting (2-10 weeks), rarely progressing to ALF or to chronicity if the infection occurs in immunosuppressed patients (HIV, preexisting liver disease). The neonates have the highest risk of developing ALF, especially if mothers become infected in the last trimester of pregnancy(13,15-19).
Hepatitis F virus (HFV) and hepatitis G virus (HGV)
HFV or HGV infection is sporadic in children. Still, it may progress to fulminant forms of infection, especially in patients with a liver transplant or preexisting liver disease(20-22).
I.2. Nonhepatotropic viruses
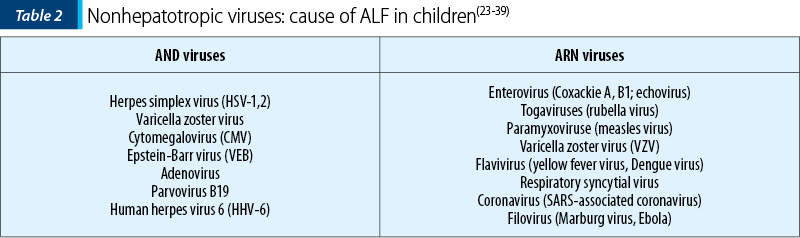
ALF can be secondary to viral infections that are not specific to the liver cell alone. The main viral agents involved are presented in Table 2.
Herpes simplex virus (HSV-1,2)
Although rare (less than 1% of ALF cases), liver damage in HSV infection can be extremely severe, with a mortality of over 85% in the absence of treatment. The only source of infection is humans; the transmission is made through close contact with infected people. In neonates, HSV is usually transmitted during delivery through an infected maternal genital tract (85%). Less often, intrauterine (5%) or postpartum transmission (10%) may account. The incidence of neonatal HSV infection varies from 1:3,000 to 1:20,000. In older children, disseminated HSV infection associated with ALF occurs with predilection in immunosuppressed hosts or with preexisting chronic liver disease (chronic hepatitis B)(23-26).
Varicella zoster virus
Varicella hepatitis predominantly affects immunosuppressed hosts, such as transplant recipients, cancer and AIDS patients, but it can also be seen in immunocompetent children(23-26).
Cytomegalovirus (CMV)
Cytomegalovirus is part of the Herpesviridae family and is also called Herpesvirus-5. It is the leading cause of congenital infection in humans, with an incidence of 0.5-2.2%. CMV infection can cause severe forms of hepatitis with ALF, especially in neonates or in children with immunosuppression (HIV/AIDS, patients receiving prolonged corticosteroids, organ transplants). In congenital CMV infection, the transmission is transplacental, intrapartum or postnatal through breast milk. In 90% of the cases, there are no clinical signs of infection in the first days after birth. In older children and teenagers, the transmission is horizontal, through direct contact with drops of infected saliva, exposure to contaminated biological products or organ transplants. Most often, CMV infection causes anicteric forms of acute hepatitis, with moderate increases in serum transaminases but with high levels of bilirubin and cholestasis enzymes, sometimes progressing to severe forms of ALF(27-30).
Epstein-Barr virus (VEB)
Epstein-Barr virus is a DNA virus included in the Herpesvirus family, being extremely widespread in the world (90-95% of the adult population is seropositive). ALF may be a complication of infectious mononucleosis in children (1/3000 cases) or it may be the only manifestation of EBV infection, occurring with predilection in immunosuppressed patients (HIV/AIDS, prolonged corticosteroid therapy, organ transplantation, malnutrition). Most often, there are forms of anicteric hepatitis that can associate high fever, pseudomembranous tonsillitis, splenomegaly, adenomegaly or thrombocytopenia(31-32).
ALF secondary to infection by other viruses, such as parvovirus, adenovirus and enterovirus, is very rare, with only a few cases described in the literature and these mainly in adults(33-39).
1.3. Bacterial, parasitic and fungal infections
Despite numerous advances in medicine and the emergence of new classes of antibiotics, the bacterial infections with systemic resonance continue to cause a high morbidity and mortality among children, in some countries being the leading cause of death in infants and young children. The liver plays the role of a large lymphatic organ in systemic bacterial infection. Hepatic impairment occurs in 20-45% of cases with systemic bacterial infection, with an incidence of ALF of 8.5%. The liver lesions are secondary to the direct action of the pathogen, toxins and mediators released. Gram-negative bacilli (BGN) are the leading infectious agents involved in sepsis-associated liver dysfunction. The hepatic injury usually occurs in the early stages of infection, therefore the association of ALF can be considered a prognostic factor in the evolution of systemic infection(40-42).
Leptospirosis, considered the most common zoonosis in humans, is caused by spirochetes from the genus Leptospira interrogans. It represents a rare cause of ALF in children, but it can determine severe forms of infection. Humans become infected through contact with mucous membranes, conjunctiva or skin lesions with contaminated soil or water from infected animals (most commonly rats). The systemic form of infection is Weil’s disease and associates ALF with cholestasis, acute renal injury, myalgia, myocarditis, pulmonary hemorrhage or adult-type respiratory distress (ARDS)(43,44).
Extremely rare, Toxoplasma gondii, a protozoan parasite, can cause ALF in immunosuppressed or transplanted children. In these cases, the infection is associated with severe systemic phenomena: fever, generalized lymphadenopathy or multiple organ failure (MSOF). It is transmitted by the fecal-oral route, the reservoir being the cats that contaminate the soil with cysts. Also, it can be transmitted from the infected pregnant woman to the fetus, primarily if she acquires the infection in the last trimester of pregnancy(45).
II. Toxic causes of ALF
Toxicity is one of the most common causes of ALF in children, with a prevalence of 20-25%. The most common toxics which cause ALF are mushrooms (Amanita phalloides) and drugs (acetaminophen, sodium valproate, tuberculostatic medication)(46-48).
Mushrooms
Mushroom poisoning represents an important cause of ALF worldwide in children and adults. In Europe, approximately 50-100 deaths/year are reported due to mushroom ingestion(46). Amanita phalloides intoxication is the most common cause of severe mushroom poisoning worldwide, being responsible for 90% of deaths due to mushroom consumption(47,49). The clinical picture of ALF in mushroom poisoning appears late, 2-4 days after ingestion, and includes jaundice, hepatic encephalopathy and severe hemorrhages(49-51).
Drugs
Drugs ingestion represents another important cause of toxic ALF in children. Drugs can cause hepatotoxicity by two mechanisms: intrinsic or dose-dependent toxicity (acetaminophen, halothane) and idiosyncratic reactions (antibiotics, nonsteroidal anti-inflammatory drugs, isoniazid). Depending on the mechanism of action, drugs determine the destruction of the hepatocyte membrane or bile ducts, activating T lymphocytes and tissue necrosis factor (TNF-alpha), altering the mitochondrial function or inducing apoptosis in the hepatocyte. Intrinsic drug hepatotoxicity is dose-dependent, independent of the genetic load of the body, and the symptoms of toxicity appear after a short period (hours, days) after ingestion. Idiosyncratic hepatotoxicity occurs in people with genetic predisposition, is dose-independent and involves immune-mediated phenomena. In this case, the manifestations of hepatotoxicity occur at a distance (weeks) from drug exposure, often associated with elements of autoimmunity, rash, fever, arthralgia or eosinophilia(52-55).
III. Immune causes
Autoimmune hepatitis (AIH)
AIH is a rare condition in children with a genetic predisposition (HLA-DR3/DR4). It is an inflammatory disease of the liver that is characterized by an increased serum IgG level (most often), the presence of specific antibodies (antinuclear antibodies – ANA, anti-smooth muscle antibodies – SMA, anti-liver kidney microsomal – LKM1), and inflammatory infiltrate in the portal space at the histopathological examination. Although a chronic disease, in over 50% of cases the onset of the disease can be acute, and in 10-11% even with ALF. The clinical picture, the family history of autoimmunity (positive in 40% of cases), the personal history of another autoimmunity (20% of cases), the treatment response and the long-term prognosis are most often similar in both types of AIH. However, the acute onset is more common in type 2 AIH, with significant coagulopathy and encephalopathy. Most often, children with AIH presenting with ALF and hepatic encephalopathy do not respond to any form of immunosuppression therapy and need urgent liver transplantation(56,57).
Neonatal hemochromatosis
Neonatal hemochromatosis, also called GALD (gestational alloimmune liver disease), is the consequence of an alloimmune gestational pathology, which consists of the deposition of extrahepatic iron in the presence of antibodies directed against fetal liver cells. The process begins around the gestational age of 12 weeks and is immune-mediated (IgG from the mother). The hepatic impairment begins intrauterinely and continues postnatally with ALF. The neonates will show signs of intrauterine distress, growth retardation, premature birth, oligohydramnios, prenatal signs of cirrhosis or ascites. In the first hours/days after birth, the manifestations of ALF will occur with jaundice, severe coagulopathy (INR>4), hypoalbuminemia, edema, hyperammonemia, hypoglycemia or with manifestations of neonatal sepsis(58-61).
IV. Metabolic causes
IV.1. Inborn errors of metabolism (IEM)
Galactosemia
It is a genetic disease with autosomal recessive transmission caused by mutations in the gene that encodes the enzymes involved in galactose metabolism. Depending on the deficient enzyme, there are three types of the disease, the most severe and the most common being the classic form (galactose-1-phosphate uridylyl-transferase [GALT] deficiency), with an incidence of 1:20,000-50,000 neonates. GALT enzyme deficiency leads to the accumulation of toxic metabolites (galactose, galactitol or galactose-1-phosphate) in almost all cells of the body, causing severe damage. In the neonates or infants, the clinical manifestations appear early, with severe ALF, acute renal injury, nonimmune hemolytic anemia, convulsions (due to severe episodes of hypoglycemia) and coma. The toxic metabolites affect the phagocytic function of neutrophils, causing severe systemic infections, often with Escherichia coli. The most severe is hepatic impairment, often with ALF. The other clinical manifestations are secondary to the accumulation of galactose-1-phosphate in the tissues, where it causes cataracts “in oil stain”, acute tubular necrosis with secondary Fanconi syndrome, or neuropsychiatric disorders. Type 2 (due to galactokinase deficiency) and type 3 (due to uridine diphosphate galactose-4 epimerase deficiency) are less common and cause milder forms of the disease(62-66).
Hereditary fructose intolerance
It is a rare autosomal recessive condition (1:20,000-30,000 neonates), caused by mutations in the ALDOB gene that reduce aldolase B activity (one of the three enzymes involved in fructose metabolism). The ingestion of fructose leads to the impossibility of its metabolism, with the accumulation of fructose-1-phosphate and the appearance of ALF phenomena, renal impairment, neurological toxicity or hypoglycemia(67).
Tyrosinemia
It is a genetic disease with an autosomal recessive transmission, caused by the reduction or absence of the activity of fumaryl acetoacetate (tyrosinemia type I) or other enzymes involved in the metabolism of tyrosine (type II and III disease). It is a rare condition, with an incidence of 1:100,000 neonates in type I; the other forms are even rarer. Tyrosine is a metabolite of phenylalanine and results from the metabolism of food proteins. The symptomatology is very severe and most often appears in the first weeks of life, secondary to the accumulation of succinylacetone in the liver and kidneys. Severe ALF, hepatosplenomegaly, ascites, coma, acute renal injury or coagulopathy are the manifestations of the disease that obscure the prognosis of these patients(68,69).
In recent years, many other inborn errors of metabolism have been involved in the etiology of ALF, such as mitochondrial respiratory disorders, fatty acid oxidation defects or urea cycle disorders(70).
IV.2. Wilson disease (WD)
Wilson disease is an autosomal recessive genetic condition in which the ATP7B gene on chromosome 13 is affected. The disease was first described in 1912, by Kinnear Wilson, as combining hepatolenticular degeneration and neuropsychic manifestations. The ATP7B gene synthesizes the ATP7B protein found in the hepatocyte, renal tubular cells, erythrocyte and neuronal cells. In hepatocytes, ATP7B plays a role in transmembrane and intrahepatocyte transport of copper and favors its incorporation into apoceruloplasmin in the Golgi apparatus to form ceruloplasmin. Mutations in the ATP7B gene cause the deposition of excess copper in cells and tissues. WD generally occurs in older children and adolescents, with a prevalence of 1:30,000-50,000 cases in Europe. The clinical manifestations are varied: hepatic manifestations, more common in children (jaundice, hepatic steatosis, hepatitis, ALF, cirrhosis, ascites), neurological manifestations, psychiatric manifestations, or ocular changes (Kayser-Fleischer ring). In adolescents, the onset may be as ALF associated with hemolytic anemia and sometimes with acute renal injury, manifestations known as fulminant WD. Most of the time, the prognosis is poor in these forms of the disease, the only therapeutic option being the emergency liver transplant(71-75).
V. Vascular causes
Although it is a very rare cause, ischemia secondary to severe hypotension, cardiac arrest or low flow syndrome can cause ALF in children. Also, sinusoidal obstruction syndrome (veno-occlusive disease), Budd-Chiari syndrome or hepatic stasis from acute heart failure represent extremely rare causes of severe liver injuries in children, being found especially among adults(1-4).
VI. Malignancies
ALF as a presentation of hemophagocytic lymphohistiocytosis (HLH) or leukemia is rare and often results from the multiorgan failure during the progression of these disorders or secondary to chemotherapy. HLH is classified as either primary (or familial HLH) or secondary (or acquired HLH) and represents a spectrum of inherited and acquired conditions secondary to an uncontrolled immune response. The diagnosis of HLH includes high fever, hepatosplenomegaly, high alkaline phosphatase, lactate dehydrogenase and abnormalities on peripheral blood film(1-4).
Etiology of ALF in children around the world
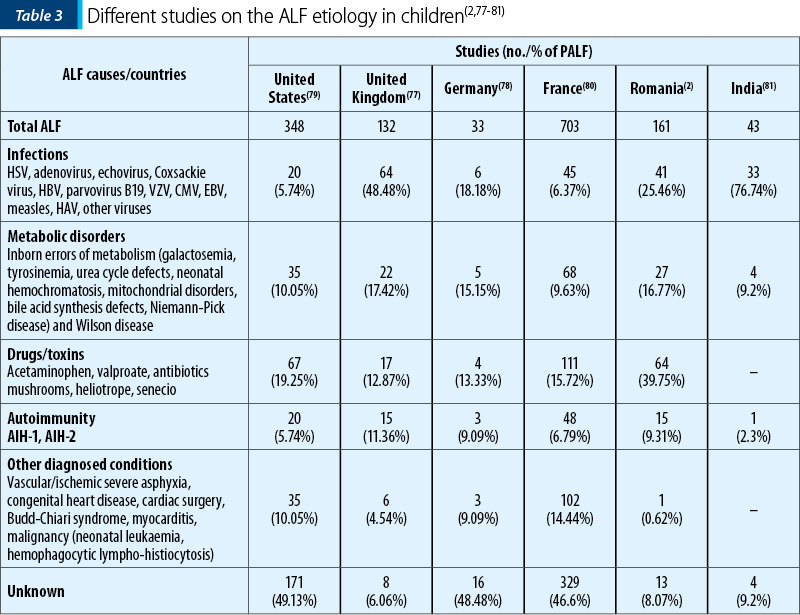
The infectious and metabolic disorders represent the leading causes in neonates and infants, while in children and teenagers, in addition to viral causes, toxic, autoimmune hepatitis or Wilson’s disease are incriminated (Table 3). Despite the numerous investigations that can be done, more than 45% of ALF cases in children remain of unknown etiology(2,77-81) – Table 3.
The management requires a multidisciplinary approach and is directed at establishing the etiology and monitoring, anticipating and managing the multisystem complications in children with ALF. Overall, the short-term outcomes are better in children than in adults, but are dependent upon the degree of encephalopathy and diagnosis.
Conclusions
In recent years, liver transplantation has increased the chance of survival in children with ALF. However, the risk of death of these patients remains high. The knowledge of ALF etiology plays a critical role in the management of such cases, as sometimes the child could benefit from specific therapies in emergency (specific diets in metabolic disorders, copper chelator in WD, administration of antidotes, liver dialysis, hemoperfusion or plasma separation in severe toxic hepatitis), thus reducing the need for transplant intervention.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
-
Squire SR. Acute Liver Failure in Children. Sem Liv Dis. 2008;28(2):153-166.
-
Grama A, Aldea CO, Burac L, et al. Etiology and Outcome of Acute Liver Failure in Children – The Experience of a Single Tertiary Care Hospital from Romania. Children. 2020;7(12):282.
-
Whitington FP, Alonso MW. Fulminant Hepatitis and Acute Liver Failure. In: Deirdre K (editor). Diseases of the Liver and Billiary System in Children, 3rd edition, Oxford, Wiley-Blackwell, 2008;92-123.
-
Gilbert PJ, Moreno BJ, Rodriguez SM. Aetiology, outcomes and prognostic indicators of paediatric acute liver failure. Anales de Pediatria. 2018;88(2):61-112.
-
Grama A, Burac L, Cainap S, et al. Acute liver failure in children: aetiology and evolution. Archives of Disease in Childhood. 2019;6:104.
-
Diniz-Santos DR, Clotildes M, Melo N, Melo FR, Silva LR. Acute liver failure complicating viral hepatitis A. Braz J Infect Dis. 2004 Apr;8(2):180-3.
-
Vallbracht A, Fleischer B, Busch FW. Hepatitis A: hepatotropism and influence on myelopoiesis. Intervirology. 1993;35(1-4):133-9.
-
Tseng YR, Wu1 JF, Kong MS, et al. Infantile Hepatitis B in Immunized Children: Risk for Fulminant Hepatitis and Long-Term Outcomes. PLoS One. 2014;9(11):e111825.
-
Zhang HW, Yin HJ, Li TY. Risk factors for acute hepatitis B and its progression to chronic hepatitis in Shanghai, China. Gut. 2008;57(12):1713–1720.
-
Wai CT, Fontana RJ, Polson J, et al. Clinical outcome and virological characteristics of hepatitis B-related acute liver failure in the United States. J Viral Hepat. 2005;12:192–8.
-
Bleich LM, Swenson ES. Prevention of neonatal hepatitis B virus transmission. Journal of Clinic Gastroenterology. 2014;48(9):765-72.
-
Squire JE, Balistreri WF. Hepatitis C infection in children and adolescents. Hepatology Communications. 2017;1(2):87-98.
-
Manka P, Verheyen J, Gerken G, Canbay A. Liver Failure due to Acute Viral Hepatitis (A-E). Visc Med. 2016;32(2):80–85.
-
Xue MM, Glenn JS, Leung DH. Hepatitis D in Children. J Pediatr Gastroenterol Nutr. 2015 Sep;61(3):271-81.
-
Fontana RJ, Engle RE, Trivedi S, et al. The role of hepatitis E virus infection in adult American patients with acute liver failure. Hepatology. 2012;56:958A–959A.
-
Davern TJ, Chalasani N, Fontana RJ, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665-1672.
-
Dalton HR, Fellows HJ, Stableforth W, et al. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429–1435.
-
Crossan CL, Simpson KJ, Craig DG, et al. Hepatitis E virus in patients with acute severe liver injury. World J Hepatol. 2014;6:426–434.
-
Hartl J, Wehmeyer MH, Pischke S. Acute Hepatitis E: Two Sides of the Same Coin. Viruses. 2016;8(11):299.
-
Sung MW, Thung SN. Hepatitis F, G, and TT Viruses. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.
-
Loginov AS, Sharafanova TI, Reshetniak Vi, et al. [HGV and TTV - new hepatitis viruses]. Ter Arkh. 2000;72(11):9–13.
-
Yang JF, Dai CY, Chuang WL, et al. Prevalence and clinical significance of HGV/GBV-C infection in patients with chronic hepatitis B or C. Jpn J Infect Dis. 2006;59(1):25–30.
-
Norvell JP, Blei AT, Jovanovic BD, Levitsk J. Herpes Simplex Virus Hepatitis: An Analysis of the Published Literature and Institutional Cases. Liver Transplantation. 2007;13(10):1428–1434.
-
Kimberlin DW. Neonatal Herpes Simplex Infection. Clin Microbiol Rev. 2004;17(1):1–13.
-
Abuhasna SD, Shihab ZM, Al Niyadi SM, et al. Neonatal Herpes Simplex Fulminant Hepatitis Successfully Treated with Acyclovir. J Clin Neonatol. 2012;1(2):87–90.
-
Verma A, Dhawan A, Zuckerman M, Hadzic N, Baker AJ, Mieli-Vergani G. Neonatal herpes simplex virus infection presenting as acute liver failure: Prevalent role of herpes simplex virus type I. J Pediatr Gastroenterol Nutr. 2006;42:282–6.
-
Chae-Yeon M, Joo Young S, Su Jin J. Characteristics and prognosis of hepatic cytomegalovirus infection in children: 10 years of experience at a university hospital in Korea. Korean J Pediatr. 2017;60(8):261–265.
-
Jensen KO, Angst E, Hetzer FH, Gingert C. Acute Cytomegalovirus hepatitis in an immunocompetent host as a reason for upper right abdominal pain. Gastroenterology. 2016;10(1):34-43.
-
Ozkan TB, Mistik R, Dikici B, Nazlioglu HO. Antiviral therapy in neonatal cholestatic cytomegalovirus hepatitis. BMC Gastroenterol. 2007;7:9.
-
Grama A, Sirbe C, Grigore M, et al. Congenital cytomegalovirus infection. Archives of Disease in Childhood. 2019;104(S3):A291.
-
Kang M, Kim T, Shim KN. Infectious Mononucleosis Hepatitis in Young Adults: Two Case Reports. Korean J Intern Med. 2009;24(4):381–387.
-
Adams LA, Deboer B, Jeffrey G, Marley R, Garas G. Ganciclovir and the treatment of Epstein-Barr virus hepatitis. J Gastroenterol Hepatol. 2006;21(11):1758-60.
-
Suchy FJ. Fulminant Hepatic Failure. In: Behrman RE, Kliegman RM, Jenson HB (editors), Nelson Textbook of Pediatrics, 17th edition, USA, Saunders, 2004;1048-1050.
-
Bihari C, Rastogi A, Saxena P. Parvovirus B19 Associated Hepatitis. Hepat Res Treat. 2013:472027.
-
Hofinut OF, Canan O, Özcay F, Bilezikci B. Adenovirus infection as possible cause of acute liver failure in a healthy child: A case report. Turk J Gastroenterol. 2008;19(4):281-283.
-
Bersani I, Auriti C, Piersigilli F, Dotta A. Neonatal acute liver failure due to enteroviruses: a 14-years single NICU experience. J Matern Fetal Neonatal Med. 2019;30:1-5.
-
Kawashima H, Ryou S, Nishimata S, et al. Enteroviral hepatitis in children, Enteroviral hepatitis in children. Pediatrics International. 2004;46(2):130-134.
-
Nobilia V, Petrinic S, Devito R. Acute liver failure following measles virus infection. Digestive and Liver Disease. 2007;39(10):A69–A70.
-
Figueiredo CA, Cordovani NTB, Castrignano SB. Acute liver failure associated with rubella virus in a child. The Pediatric Infectious Disease Journal. 2010;29(6):573-574.
-
Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33(6):498–510.
-
Weiss YG, Bellin L, Kim PK, et al. Compensatory hepatic regeneration after mild, but not fulminant, intraperitoneal sepsis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G968–73.
-
Balasubramanian M, Agrawal R, Malik S, Lad D. The Liver in Sepsis in Early Childhood: An Autopsy Study. GJRA. 2007;6(6):2277–8160.
-
Mendoza HR, Sencion-Paulino C, Torres-Rosario CJ, Perez C, Koenig E. Anticuerpos IgM leptospirosicos en casos de hepatitis infecciosa aguda (HIA) en ninos [IgM Leptospira antibodies in acute infectious hepatitis cases in children]. Arch Domin Pediatr. 1991;27(2):39-41 [Spanish].
-
Gompf SG, Velez AP, Green-McKenzie J. Leptospirosis. Available at: https://emedicine.medscape.com/article.
-
Botterel F, Ichai P, Feray C, et al. Disseminated Toxoplasmosis, Resulting from Infection of Allograft, after Orthotopic Liver Transplantation: Usefulness of Quantitative PCR. Journal of Microbiology. 2002;40(5):1648-50.
-
Grama A, Aldea CO, Burac L, et al. Acute liver failure secondary to toxic exposure in children. Archives of Medical Science. 2022;18(1).
-
Colleti J Jr, Azevedo RT, de Calvalho WB. Pediatric Acute Liver Failure: Current Perspectives. Liver Research. 2017;2(1):14-15.
-
Grama A, Burac L, Aldea CO, et al. Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children. Diagnostics. 2020;10(5):278.
-
Santi L, Maggioli C, Mastroroberto M, Tufoni M, Napoli L, Caraceni P. Acute Liver Failure Caused by Amanita phalloides Poisoning. International Journal of Hepatology. 2012;2012:487480.
-
Mengs U, Pohl RT, Mitchell T. Legalon® SIL: the Antidote of Choice in Patients with Acute Hepatotoxicity from Amatoxin Poisoning. Curr Pharm Biotechnol. 2012;13(10):1964-1970.
-
Broussard NC, Aggarwal A, Lacey RS, et al. Mushroom poisoning – from diarrhea to liver transplantation. American Journal of Gastroenterology. 2001;96(11):3195–3198.
-
Dhawan A. Acute Liver Failure in children and adolescents. Clin Res Hepatol Gastroenterol. 2012;36(3):278-283.
-
Robert A, Ganey P. Intrinsic versus Idiosyncratic Drug-Induced Hepatotoxicity – Two Villains or One. J Pharmacol Exp Ther. 2010;332(3):692–697.
-
Pandit A, Sachdeva T, Bafna P. Drug-Induced Hepatotoxicity: A Review. Journal of Applied Pharmaceutical Science. 2012;02(05):233-243.
-
Grama A, Bizo A, Delean D, Aldea C, Bulata B, Pop TL. Drug-induced acute liver failure in children. European Journal of Pediatrics. 2017;176(11):1469.
-
Pathak S, Kamat D. Autoimmune Hepatitis in Children. Pediatric Annals. 2018;47(2):e81-e86.
-
Mieli-Vergani G, Vergani D. Autoimmune paediatric liver disease. World J Gastroenterol. 2008;14(21):3360–3367.
-
Taylor SA, Whitington PF. Neonatal acute liver failure. Liver Transpl. 2016;22:677-685.
-
Lopriore E, Mearin LM, Oepkes D, et al. Neonatal hemocromatosis: management, outcome, and prevention. Prenatal Diagnosis. 2013;33(13):1221-1225.
-
Bonilla S, Prozialeck JD, Malladi P, et al. Neonatal iron overload and tissue siderosis due to gestational alloimmune liver disease. J Hepatology. 2012;56(6):1351-1355.
-
Busoms CM, Bernabeu JQ, Carpi JM. Neonatal hemochromatosis: Another entity that is no longer orphan. Advances in the diagnosis and management of the main cause of neonatal acute liver failure. An Pediatr (Barc). 2015;83(3):e1-288.e3.
-
Dobrowolski SF, Banas RA, Suzow JG, et al. Analysis of Common Mutations in the Galactose-1-Phosphate Uridyl Transferase Gene. J Mol Diagn. 2003;5(1):42–47.
-
Grama A, Blaga L, Nicolescu A, et al. Novel Mutation in GALT Gene in Galactosemia Patient with Group B Streptococcus Meningitis and Acute Liver Failure. Medicina. 2019;55(4):91.
-
Elsas LJ, Langley S, Steele E, et al. Galactosemia: a strategy to identify new biochemical phenotypes and molecular genotypes. Am J Hum Genet. 1995;56:630-639.
-
Elsas LJ, Lai K. The molecular biology of galactosemia. Genet Med. 1998;1(1):40-48.
-
Grama A, Pop I, Tita G, et al. P121 Galactosemia presented as a fulminant liver failure and group B streptococcus (GBS) sepsis. Archiv Dis Childhood. 2017;102:A81.
-
Roth KS. Hereditary Fructose Intolerance (HFI) (Fructose 1-Phosphate Aldolase Deficiency). Pediatrics: Genetics and Metabolic Disease. 2017.
-
Roth KS. Tyrosinemia Pediatrics: Genetics and Metabolic Disease în Maria Descartes, Luis O Rohena, editors. Drugs and Diseases, 2017. Available at: https://emedicine.medscape.com/article/949816. Accesed: Oct. 2018.
-
Rashad MM, Nassar C. Tyrosinemia Type 1: A case report. Sudan J Paediatr. 2011;11(1):64–67.
-
Taylor SA, Whitington PF. Neonatal acute liver failure. Liver Transpl. 2016;22:677-685.
-
Tanner S. Disorders of Copper Metabolism. In: Deirdre K (editor). Diseases of the Liver and Biliary System in Children, 3rd edition, Oxford, Wiley-Blackwell, 2008;328-351.
-
Grama A, Bizo A, Pop T, et al. Fulminant liver failure as the presentation in Wilson disease in adolescence. 7th Europaediatrics, EPA-UNEPSA. 2015.
-
Socha P, Janzyk W, Dhawan A, et al. Wilson’s Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(2):334-342.
-
Tian Y, Gong GZ, Yang X, Peng F. Diagnosis and management of fulminant Wilson’s disease: a single center’s experience. World J Pediatr. 2016;12(2):209-14.
-
Arthur JM, Fleming R, Thistle JL, et al. Diagnosis of Wilson’s Disease Presenting as Fulminant Hepatic Failure. Gastroenterology. 1983;84(1):161-7.
-
Lin S, Ying L, Long J, Qichuan L, Fangwan Y. Acute liver failure caused by hemophagocytic lymphohistiocytosis in adults. Medicine. 2016;95(47):pe5431.
-
Dhawan A. Etiology and Prognosis of Acute Liver Failure in Children. Liver Transplantation. 2008;14:S80-S84.
-
Kathemann S, Bechmann L, Sowa JP, et al. Etiology, outcome and prognostic factors of childhood acute liver failure in a German Single Center. Annals of Hepatology. 2015;14(5):2015.
-
Squires RH Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652-658.
-
Devictor D, Tissieres P, Afanetti M, Debray D. Acute liver failure in children. Clinics and Research in Hepatology and Gastroenterology. 2011;35(6-7):430-437.
-
Kaur S, Kumar P, Kumar V, Kumar S, Kumar A. Etiology and Prognostic Factors of Acute Liver Failure in Children. Indian Pediatr. 2013;50(7):677-679.
Articole din ediţiile anterioare
Intoleranţa ereditară la fructoză: capcană de diagnostic la sugar?
Hereditary fructose intolerance (HFI) – also called fructose-1-phosphate aldolase deficiency – is a rare inherited metabolic disorder characterized...
Particularităţi şi aspecte comune ale intoxicaţiilor în populaţia pediatrică şi cea adultă
Intoxicaţiile acute la grupa de vârstă pediatrică reprezintă una dintre principalele cauze de mortalitate şi de morbiditate, cu impact major în cee...
Sleep-related breathing disorders in the pediatric patient with Prader-Willi syndrome
Sindromul Prader-Willi (PWS) este o boală genetică rară, întâlnită cu o incidenţă de 1:15000 de nou-născuţi, identificată la toate rasele şi car...
Evaluarea managementului respirator la copiii cu distrofie musculară Duchenne
Distrofia musculară Duchenne este o boală genetică cu transmitere X-linkată, care afectează gena implicată în sinteza distrofinei. Nivelurile ...