Trisomy 18 is a rare genetic disorder (1/6000 liveborn infants), with controversial issues nowadays regarding the intensive medical interventions, as 8-12% of children survive beyond the first year, with a satisfactory quality of life. The clinical features are specific and the cardiac malformations usually confer the gravity of the case, with high mortality during the first weeks after delivery and during the first year of life (about 90%). We present the case of a girl with trisomy 18, with complex cardiac malformation, for which no surgery was indicated, who died at the age of 5 months due to acute pneumonia.
Trisomia 18 – cauză rară de malformaţie cardiacă complexă
Trisomy 18 – a rare disease with complex cardiac malformation
First published: 22 octombrie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.59.3.2020.3901
Abstract
Rezumat
Trisomia 18 este o boală genetică rară (1/6000 de nou-născuţi vii), existând controverse în prezent în ceea ce priveşte intervenţiile medicale intensive indicate, datorită supravieţuirii în proporţie de 8-12% a copiilor şi după vârsta de 1 an, având o calitate a vieţii mulţumitoare. Trăsăturile clinice sunt caracteristice, iar malformaţiile cardiace conferă, de obicei, gravitatea cazului, cu o mortalitate mare în primele săptămâni după naştere şi în primul an de viaţă (aproximativ 90%). Prezentăm cazul unei fetiţe cu trisomie 18, cu malformaţie cardiacă importantă, considerată a nu impune intervenţie chirurgicală, care a decedat la vârsta de 5 luni, în contextul unei pneumonii acute.
Abbreviations: GA = gestational age; BW = birth weight; BL = birth length; HC = head circumference; CCM = congenital cardiac malformation; VSD = ventricular septal defect; OS ASD = ostium secundum atrial septal defect; RV = right ventricle; RVH = right ventricle hypertrophy; PH = pulmonary hypertension; TFUS = transfontanelar ultrasound; AF = anterior fontanelle; PF = posterior fontanelle; VM = vesicular murmur; HR = heart rate; RUL = right upper lobe; GER = gastroesophageal reflux
Introduction
Trisomy 18 (Edwards syndrome), a rare genetic condition, recognized since 1960, is the second most common autosomal trisomy, after trisomy 21(1). Due to the third copy of chromosome 18, there is an extra genetic material, leading to developmental anomalies and to life-threatening malformations which explain the short life span for the majority of cases(2). Mosaic trisomy 18 accounts for 5% of the cases, meaning that they have only some of the body cells affected, hence the different grades of the disease’s severity in these children(3).
The prevalence of the syndrome is about 1 in 6000 live-born infants, with a preponderance in females (sex ratio 3:1); in reality, the number of cases is higher because many embryos and fetuses don’t survive until birth. There seems to be a relation between the disease and older mothers (the median age of mother was 32 years old)(4).
Both complete trisomy 18 and mosaic trisomy 18 are not inherited. Instead, partial trisomy – meaning that an unaffected person carry a balanced translocation between chromosome 18 and another chromosome – has the risk to be transmitted to the child.
Over 130 abnormalities have been described for this syndrome, among them decreased fetal activity, intrauterine growth retardation, preterm or post-term birth, feeding difficulties, neonatal hypotonia followed by hypertonia, muscular atrophies, specific craniofacial features with proeminent occiput, mycrocephaly (and to a lesser extent hydrocephaly), large fontanels, narrow bifrontal diameter, low-set ears, narrow eyelid folds, epicanthus, small mouth, micrognathia, ogival palate, clenched hand, with overlap of the 2nd and 5th fingers over the 3rd and 4th, rocker bottom feet, syndactyly of certain toes, hypoplastic nails (mainly in the fifth finger), mental retardation, along with abnormalities of other organs (lungs, heart, digestive system, kidneys, genitals)(5,6).
Children frequently need resuscitation at birth and then they can present with apnea(1). They need nasogastric tube feeding and have a slow postnatal growth. About half of the cases die in the first week and 90% of them die during the first year of life due to cardiac, renal or neurological complications, or to recurrent infections(7). Between 8% and 12% will survive the first year of life, usually with major developmental difficulties and mental retardation(8).
There is no treatment that can cure trisomy 18; the current guidelines recommend that the management should be tailored to each individual case and the family must know and be involved in the management options(9,10). These include from palliative care to corrective surgeries(11,12).
Case presentation
A 2-month-old baby girl was discharged from the maternity hospital and admitted to a foundation for special health care needs.
Her mother was a 15-year-old girl and her father was 28 years old, not married, healthy, from the rural area. The mother had no medical control during pregnancy and had a spontaneous vaginal delivery at 33 weeks of gestational age. The child birth weight was 2000 grams, the length was 45 cm, the head circumference was 31 cm, the Apgar score was 7, and she received the BCG vaccine.
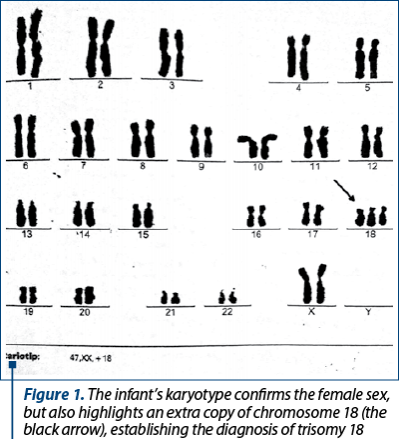
The discharge diagnosis was homogenous trisomy 18 (karyotype 46,XX,+18) – Figure 1, complex CCM (cardiac ultrasound with moderate-to-large perimembranous VSD; small OS ASD; marked tricuspid valve dysplasia; moderate-to-large left ventricular outflow tract obstruction; moderate-to-large pulmonary valve stenosis; large supravalvular pulmonary stenosis; mild PH; RVH), bilateral congenital talipes equinovarus and preterm-infants jaundice. TFUS and abdominal ultrasound showed no evident malformations. She was fed by nasogastric tube, she had the third long leg plaster cast for bilateral clubfoot, and she was on chronic treatment with captopril and furosemide.
The physical examination showed an infant with mediocre health condition, pallor with mild circumoral cyanosis, colder extremities, thin subcutaneous fat, weight 2900 grams (including the cast), length 48 cm, head circumference 34.5 cm, facial dysmorphism with mycrocephaly, open skull sutures and wide fontanelles (AF=4/3 cm and PF=3/3 cm), dolichocephaly with protruding occiput, micrognathia, synophrys, single transverse palmar crease at both hands, clenched hand, with overlap of the 2nd and 5th fingers over the 3rd and 4th, syndactyly of the 2nd, 3rd and 4th toes at both feet, pectus excavatum, flared and widened ribs, tachypnea (60 bpm at rest) with polypnea, suprasternal and intercostal retractions, thoracoabdominal asynchrony, respiratory murmur with intermittent bilateral fine crackles, enlarged area at cardiac percussion, HR=140 bpm, rhythmic heart sounds, systolic murmur of grade IV/6 audible over the entire precordium and along the right sternal border, with radiation to the back, soft abdomen with diastasis recti, lower border of the liver at 1.5 cm from the right costal margin, mild clitoral hypertrophy, and labia majora not covering the labia minora.
At 3 months old, she was admitted to the hospital with acute pneumonia for 10 days. At that moment, chest X-ray showed enlarged heart and increased interstitial bilateral perihilar and hilio-basal marking on the right side (Figure 2). She recovered slowly with intravenous cefuroxime initially, and then with meropenem.
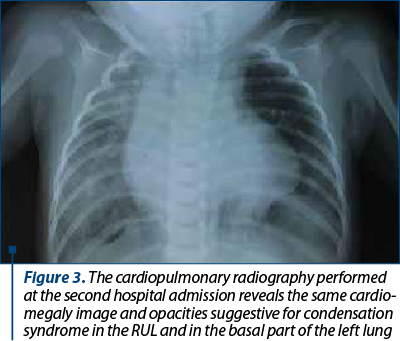
At 5 months old, she was admitted again to the hospital with a new episode of acute pneumonia, with fever and dyspnea after vomiting. At the beginning, the evolution was good with oxygen and intravenous cefuroxime plus gentamicine, but after 24 hours the respiratory distress aggravated and she was transferred in an emergency hospital, where she died after another 24 hours. The second chest X-ray showed cardiomegaly and pulmonary infiltrates, indicating a condensation syndrome in the RUL and in the basal part of the left lung (Figure 3).

Discussion
We presented a typical case of trisomy 18, a premature born infant with intrauterine growth retardation, particular phenotype, feeding difficulties, failure to thrive, psychomotor retardation and complex cardiac malformation. The particularities of this case are a minor mother and the absence of pregnancy follow-up, which explain the missing of the prenatal diagnosis and the possibility of discussion with the obstetrician about the continuation or the termination of the pregnancy. The fact that the child was hospitalized for two months at the maternity hospital and after that was admitted to a socio-medical center specialized in palliative care, instead of being taken home, explains probably that she survived until 5 months old. The severity of the case consists of inoperable complex cardiac malformation in a dystrophic infant along with feeding difficulties with possible GER, which are all risk factors for respiratory infections.
During her 5-month life, the child got six consecutive long-leg plaster casts for bilateral clubfoot which made more difficult the mobilization and daily care, the appreciation of weight gain, leading to lesions of the leg skin and the impossibility to use superficial veins for intravenous treatments in the hospital. According to the medical literature, casting for clubfoot deformity in these children is not recommended, because the method didn’t prove useful.
Conclusions
Trisomy 18 is a rare disease with a severe prognosis, but with specific features that can suggest the diagnosis which, of course, must be confirmed by genetic tests. This was the reason why we presented this case from our practice.
We must underline again how important are the prenatal evaluations in order to find out precociously the severe life-threatening congenital malformations.
We also emphasize the importance of palliative care team from maternities and children hospitals involved in counseling for parents and – why not – for doctors working and dealing with these cases, with poor prognosis, for which the complexity and intensity of the medical interventions are still sensible and disputed topics.
Bibliografie
-
Cereda A, Carey JC. The trisomy 18 syndrome. Orphanet J Rare Dis. 2012;7:81.
-
Acharya K, Leuthner S, Clark R, et al. Major Anomalies and Birth Weight Influence NICU Interventions and Mortality in Infants with Trisomy 13 or 18. J Perinatol. 2017;37(4):420–426.
-
Peron A, Carey JC (November 2014). The Molecular Genetics of Trisomy 18: Phenotype-Genotype Correlations. In: eLS.John Wiley & Sons, Ltd: Chichester. http://www.els.net [DOI: 10.1002/9780470015902.a0025246].
-
Mithilesh KL. Trisomy 18. Available at: https://emedicine.medscape.com/article/943463-overview. Updated: Oct 26, 2016.
-
Jones KL, Smith DW. Smith’s Recognizable Patterns of Human Malformation, Edition 6. Philadelphia: Elsevier Health Sciences, 2006.
-
Geormăneanu C, Geormăneanu M. Introducere în Genetica Pediatrică. Bucureşti, Editura Medicală, 1986.
-
Boghossian NS, Horbar JD, Carpenter JH, et al. Major Chromosomal Anomalies among Very Low Birth Weight Infants in the Vermont Oxford Network. J Pediatr. 2012;160(5):774–780.e11.
-
Pearson A. Never say never about our child. BMJ. 2015;350:h1246.
-
Boghossian NS, Hansen NI, Bell EF, et al. Mortality and Morbidity of VLBW Infants with Trisomy 13 or Trisomy 18. Pediatrics. 2014;133(2):226–235.
-
Wilkinson D, de Crespigny L, Xafis V. Ethical language and decision-making for prenatally diagnosed lethal malformations. Semin Fetal Neonatal Med. 2014;19(5):306–311. Correction in: Semin Fetal Neonatal Med. 2015;20(1):64.
-
Peterson JK, Kochilas LK, Catton KG, et al. Long-Term Outcomes of Children With Trisomy 13 and 18 After Congenital Heart Disease Interventions. Ann Thorac Surg. 2017;103(6):1941–1949.
-
Kosho T, Carey JC. Does medical intervention affect outcome in infants with trisomy 18 or trisomy 13? Am J Med Genet. 2016 Apr; 170A(4):847–849.
Articole din ediţiile anterioare
Medicaţia antitermică şi astmul bronşic la copil
Utilizarea medicaţiei antitermice la pacienţii cu astm bronşic a generat unele controverse, cunoscute fiind din literatură interferenţele cu patoge...
Recomandări actuale în managementul şi urmărirea astmului bronşic la copil
Astmul bronşic reprezintă cea mai frecventă boală cronică a copilăriei şi principala cauză de morbiditate, având drept consecinţe absenteism şcolar...
Corelaţii clinico-epidemiologice la copiii cu manifestări alergice – studiu cazuistic
Manifestările alergiei la vârsta pediatrică sunt diverse, fiind oglinda clinică a unui spectru extins de patologii. Incidenţa alergiei la copil est...
Actualităţi în diagnosticul hepatitei autoimune la copil
Tipurile de boli hepatice autoimune recunoscute la populaţia pediatrică sunt: hepatita autoimună (AIH), colangita sclerozantă autoimună (...