Psoriasis is no longer defined just as a dermatological disease; it is a chronic, inflammatory, immune-mediated disease, with remissions and exacerbations, with long-term treatments, plus a multitude of associated comorbidities, including low quality of life. In the case of pregnant women affected by psoriasis, the condition might improve in some cases and worsen in others. In cases of aggravation of the symptoms and comorbidities during pregnancy, physicians should weigh the benefits of psoriasis treatment with the possible risks to the mother and fetus.
Sarcina asociată cu psoriazis – rezultate negative şi tratament
Pregnancy associated with psoriasis – adverse outcomes and treatment
First published: 23 iunie 2018
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Peri.2.2.2018.1809
Abstract
Rezumat
Psoriazisul nu mai este definit doar ca o boală dermatologică, ci este o boală cronică, inflamatorie, mediată imunologic, cu remisiuni şi exacerbări, cu tratamente pe termen lung, plus o multitudine de comorbidităţi asociate, incluzând calitatea scăzută a vieţii. În cazul femeii gravide afectate de psoriazis, această afecţiune s-ar putea îmbunătăţi în unele cazuri ori s-ar putea agrava în altele. În cazurile de agravare a simptomelor şi a comorbidităţilor în timpul sarcinii, medicii trebuie să cântărească beneficiile tratamentului psoriazisului cu posibilele riscuri asupra mamei şi fătului.
Introduction
Psoriasis is a chronic inflammatory disease, expressed through remissions and exacerbations, and it can be triggered at any age and affects both sexes equally(1,2).
Psoriasis is associated with a multitude of comorbidities, such as: cardiovascular disease, dyslipidaemia, diabetes mellitus, metabolic syndrome, significant vitamin D deficiency(3,4).
One study showed that in approximately 25% of cases, psoriasis did not alter the progression during pregnancy. Also, improvements were seen in 50% of the patients studied, while it worsened in 25% of the cases(5).
Pregnancy affects psoriasis – more than 50% of patients with psoriasis during pregnancy describe an improvement in skin involvement. Studies have shown an improvement in psoriasis during pregnancy from the 10th week to the 20th week, but there has been noticed an worsening of psoriasis postpartum, which correlated with the increase in postpartum stress(6).
Hormonal growths of oestrogen and progesterone inhibit T cell activity and response, and can be the factor responsible for improving psoriasis during pregnancy. Since there is a shift from TH1 to TH2 immunity during pregnancy, autoimmune diseases such as psoriasis, classified as TH1-mediated, tend to improve. The stimulation of KC proliferation and the promotion of angiogenesis(7) may be responsible for the worsening of psoriasis during pregnancy in some patients.
A particular problem is pustular psoriasis in pregnancy, a condition that endangers both the mother and the foetus, initially classified as single and separate dermatosis of the pregnancy, usually occuring in the third trimester of pregnancy and being characterized by large-scale coalescence pustules, desquamation and systemic symptoms; pregnancy treatments include high-dose of corticosteroids, cyclosporine, narrow-band ultraviolet B, systemic antibiotics, and close monitoring of mother and foetus, with appropriate laboratory studies during pregnancy and postpartum(8,9).
There is a relationship between the occurrence of complications, low birth weight, premature birth and the severity of psoriasis. The higher the severity, the worse the evolution during pregnancy(10,11,12).
For ethical reasons, there is very little data on the treatment of psoriasis in pregnancy, most studies being from other inflammatory diseases that use the same therapy as psoriasis, such as arthritis and bowel disease.
The relationship between psoriasis and adverse pregnancy outcomes
Risk of low birth weight. Ya-Wen Yang showed in his study on 1463 mothers with psoriasis that the odds of low birth weight for women with severe psoriasis were 1.40 times greater than those of mothers without psoriasis (95% confidence interval; 1.04-1.89), but this hypothesis could not be confirmed in the case of mild psoriasis(13).
Preterm birth. Looking at psoriasis as a complex inflammatory disease, we can consider that inflammation triggers an inappropriate immune response which can lead to an increased risk of premature delivery. A meta-analysis on the association between TNF-a and premature delivery indicated increased TNF-alpha concentrations in amniotic fluid at premature delivery compared to term delivery(14). The TNF(-488)A and LTA(IVS1-82)C variants (AGG and GAC haplotype) were associated with an increased risk of preterm birth(15).
Caesarean section. Ben-David et al. confirmed the hypothesis in his study. Psoriasis was found as an independent risk factor for caesarean delivery (OR=4.1; 95% CI; 2.3-7.5; p<0.001)(16).
Preeclampsia or eclampsia. Xinaida T. Lima et al. found a relatively small number of pregnancies in which women developed preeclampsia/eclampsia: 9 (5.7%) in the psoriasis group and 26 (5.2%) in the non-psoriasis group, from a total of 412 women included in the analysis(17).
Spontaneous abortion. Studies to date have been contradictory; there is the possibility of triggering spontaneous abortion in women with porosity. More studies are needed to determine the extent to which psoriasis affects spontaneous abortion.
Therapy of psoriasis in pregnancy
Doctors are facing the problem of psoriasis therapy in women who want to become pregnant, in pregnant women, but also after birth, especially in nursing women.
Usually, pregnant women are excluded from clinical trials; as a result, there is very little data on maternal and foetal safety during psoriasis therapy.
Physicians have to balance the effects of inflammatory skin disease with the risks for the mother and the foetus during treatment.
Because a large proportion of pregnant women claim to have improvements in psoriasis during pregnancy, the main problem represents those cases with worsening symptoms, where topical therapy is insufficient.
Topical therapy
In general, topical therapy may be used; some topical agents require limitation, while others are contraindicated (Table 1).
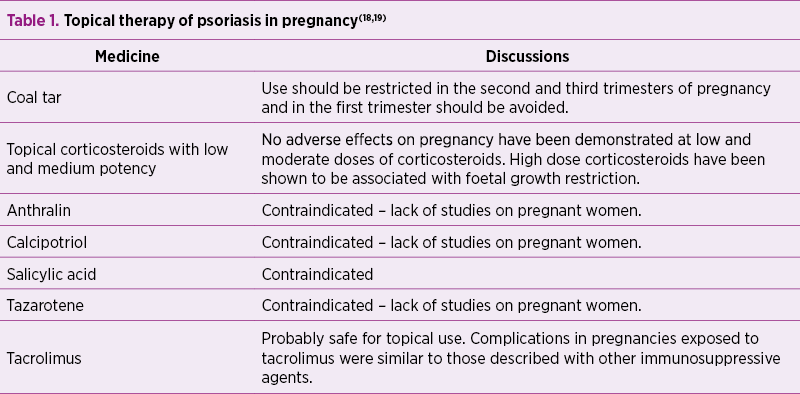
A study of 23 pregnant women treated with coal tar showed a rate of 26% spontaneous abortions and 4% congenital disorders(20).
The exposure to potent topical corticosteroids before and during pregnancy was associated with foetal growth restriction(21).
Salicylic acid is controversial in terms of pregnancy, most authors contraindicate it, while some authors believe that its use on small surfaces is safe, yet there are no studies to certify this(18,22).
A study of 100 pregnant women treated with tacrolimus showed that 71 progressed to birth, 24 were discontinued, two were ongoing and three were lost from the follow-up; the mean gestational age was 35 weeks, and 59% of the deliveries were premature. Birth weight was appropriate for gestational age in most cases, and the most common complications in the newborn were hypoxia, hyperkalaemia and renal dysfunction(23).
UVB phototherapy
The treatment with nbUVB or broadband UVB phototherapy appears to be safe in pregnancy, especially in cases where large areas of skin are affected.
It is necessary to monitor the folic acid and supplement it during the treatment with nbUVB(24).
Overheating in the first trimester of pregnancy (28 weeks) by UVB treatment should be avoided due to the risk of neuronal tube defect(19).
Topical PUVA and systemic PUVA have not yet been sufficiently investigated for the treatment of pregnant women, and are mainly contraindicated due to a lack of data.
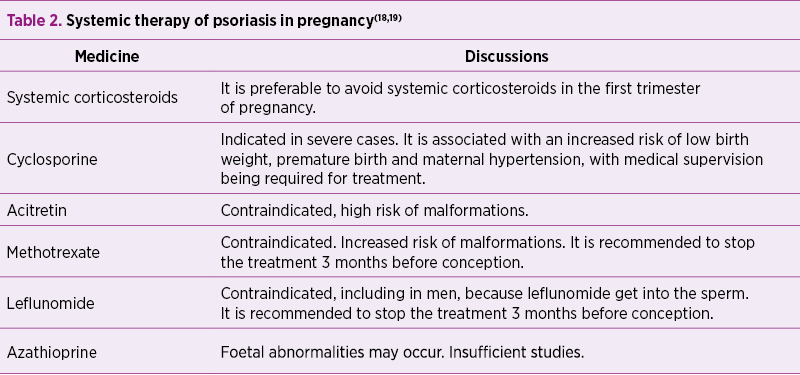
Systemic therapy
A prospective study and meta-analysis on infants exposed to corticosteroids in the first trimester found that there was an overall 3-fold increased risk of cleft palate(25).
Corticosteroids during pregnancy may be indicated when there is a risk of metabolic complications, such as persistent erythroderma not responding to other treatment and generalized pustular psoriasis(26).
Long-term use of corticosteroids can lead to risk of prematurity, restricting intrauterine growth and a high frequency of maternal adverse events such as hypertension, diabetes mellitus and osteoporosis(27).
There are limited data on the effect of ciclosporin in pregnant psoriasis patients; the drug crosses the placental blood barrier gradually to reach 10% to 50% of the maternal plasma concentration; most of the studies are on transplant patients, the dosages being much higher than in psoriasis(28).
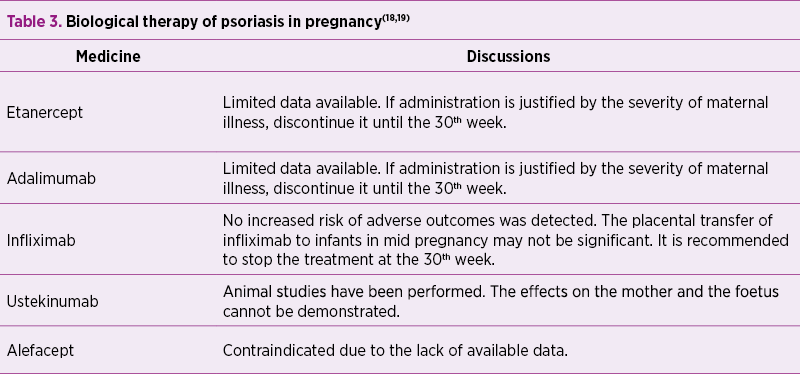
Biological therapy
Usually, biological therapy is discontinued as soon as pregnancy occurs, generally during the first trimester of pregnancy.
The expression of the foetus to biological agents during pregnancy depends on the placental transport of immunoglobulin G (IgG), which is the only major class of antibodies to be transported to the human placenta. Foetal IgG values in umbilical vein are low in the first two trimesters of pregnancy and do not exceed maternal IgG levels until the beginning of the third trimester, when active transport of transplacental IgG molecules increases rapidly(29).
Of the four subclasses of IgG (G1-G4), IgG1 is the most efficiently transported, followed by IgG4, IgG3 and IgG2, respectively(30).
Adalimumab and infliximab are both IgG1 immunoglobulins. Etanercept is also a fusion protein that contains a portion of IgG1 Fc, but with less transplacental transport than adalimumab or infliximab(31).
A relatively large study of 131 pregnancies exposed to infliximabin (the Centocor Safety Database) did not find an increased risk of negative results such as spontaneous abortions, therapeutic terminations pregnancy and congenital malformations compared to the general population(32).
The British Registry documented 130 pregnancies in 118 women, classifying them as: women exposed to anti-TNF-a drugs at the conception, women exposed to past anti-TNF-a drugs and the control group; the resulting spontaneous abortion rate was higher in the first batch compared to the second batch (24% and 17%, respectively)(19,33).
Conclusions
Due to insufficient clinical studies on the treatment of psoriasis in pregnancy, there is no treatment guide. The doctor’s decision regarding the treatment in these cases is a difficult one, and it is necessary to balance the risks and benefits of the treatment of psoriasis in pregnancy. Pregnancy outcomes depend on the severity of psoriasis and the treatment for it. It is very important for the patient to be monitored by a multidisciplinary team. The need for more studies and for the development of a treatment guide is obvious.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Stănescu AMA. Psoriazisul. În: Dumitru M. Esenţialul în medicina de familie, ediţia a 3-a. Amaltea, Bucureşti. 2016; 384-388.
3. Stănescu AMA, Matei A, Grăjdeanu IV, Appiah EA, Paparău C, Giurcăneanu C. Asocierea între psoriazis şi sindromul metabolic, corelată cu deficitul vitaminei D în ambele afecţiuni. Revista Medicală Română. 2016;1: 81-85.
4. Stănescu AMA, Goanţă AM, Ignătescu R, Appiah EA, Grăjdeanu IV, Ioniţă L. Aspecte comparative la om şi animal în diagnosticul sindromului metabolic şi disfuncţiei metabolice asociate obezităţii. Revista Practica Medicală. 2017;12, 4(53): 250-255.
5. Sorin D, Pavlovsky L, David M. Psoriasis in Pregnancy. Curr Derm Rep. 2012; 1: 209.
6. Murase JE, Chan KK, Garite TJ, Cooper DM, Weinstein GD. Hormonal Effect on Psoriasis in Pregnancy and Post Partum. Arch Dermatol. 2005;141(5):601–606.
7. Lin X, Huang T. Impact of pregnancy and oestrogen on psoriasis and potential therapeutic use of selective oestrogen receptor modulators for psoriasis. J Eur Acad Dermatol Venereol. 2016; 30: 1085-1091.
8. Stănescu AMA, Grăjdeanu IV, Diaconu C, Iancu MA, Stefani C. Evoluţia psoriazisului prenatal şi postnatal, afectarea fătului, modificări imune şi hormonale, tratament. Practica Medicală. 2018; Vol.13, Nr. 1(54): 36-40.
9. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018; 26;10:109-115.
10. Stănescu AMA, Diaconu C, Iancu MA, Bejan GC, Stefani C, Grăjdeanu IV. Psoriazisul şi bolile cardiovasculare: Actualităţi în cercetarea medicală. Revista Medicală Română. 2018; Vol LXV, Nr. 1: 41-44.
11. Stănescu AMA, Grăjdeanu IV, Appiah EA, Bejan GC. Abordare diferenţiată a psoriazisului în funcţie de vârsta pacientului. Practica Medicală. 2017; Vol. 12, Nr.4(53): 204-207.
12. Stănescu AMA, Grăjdeanu IV, Bejan GC, Iancu MA, Appiah EA, Peagu R. Sindromul metabolic în raport cu nivelul seric al vitaminei D corelat cu afectarea cutanată. Revista Medicală Română. 2017; Vol. LXIV, Nr. 4, 300-304.
13. Yang YW, Chen CS, Chen YH, Lin HC. Psoriasis and pregnancy outcomes: A nationwide population-based study. Journal of the American Academy of Dermatology. 2011; 64, 1 :71-77.
14. Menon R, Merialdi M, Betran AP, et al. Analysis of association between maternal tumor necrosis factor-alpha promoter polymorphism (−308), tumor necrosis factor concentration, and preterm birth. Am J Obstet Gynecol. 2006;195, pp. 1240-1248.
15. Holst D, Garnier Y. Preterm birth and inflammation − The role of genetic polymorphisms. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2008;141, 1: 3-9.
16. Ben-David G, Sheiner E, Hallak M, Levy A. Pregnancy outcome in women with psoriasis. J Reprod Med. 2008; 53, pp. 183-187.
17. Lima XT, Janakiraman V, Hughes MD, Kimball AB. The Impact of Psoriasis on Pregnancy Outcomes. Journal of Investigative Dermatology. 2012; Volume 132, Issue 1, Pages 85-91.
18. Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012; 67, pp. 459-477.
19. Kurizky PS, Ferreira C de C, Nogueira LSC, da Mota LMH. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. Anais Brasileiros de Dermatologia. 2015; 90(3):367-375.
20. Franssen ME, van der Wilt GJ, de Jong PC, Bos RP, Arnold WP. A retrospective study of the teratogenicity of dermatological coal tar products. Acta DermVenereol. 1999; 79:390-1.
21. Chi CC, Wang SH, Kirtschig G, Wojnarowska F. Systematic review of the safety of topical corticosteroids in pregnancy. J Am Acad Dermatol. 2010; 62:694-705.
22. Bandoli G, Johnson DL, Jones KL, Lopez Jiminez J, Salas E, Mirrasoul N, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334–339.
23. Kainz A, Harabacz I, Cowlrick IS, Gadgil SD, Hagiwara D. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000; 70:1718-21.
24. Babalola O, Strober BE. Management of psoriasis in pregnancy. Dermatol Ther. 2013; 26, pp. 285-292.
25. Park-Wyllie L, Mazzotta P, Pastuszak A, Moretti ME, Beique L, Hunnisett L, Friesen MH, Jacobson S, Kasapinovic S, Chang D, Diav-Citrin O, Chitayat D, Nulman I, Einarson TR, Koren G. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000; Dec. 62(6):385-92.
26. Griffiths CEM, Camp R, Barker JNWN. Psoriasis. In: Burns TBS, Cox N, Griffiths C, (editors). Rook’s Textbook of Dermatology. Italy: Blackwell Science. 2004; pp. 35.46-35.47.
27. Ostensen M, Forger F. How safe are anti-rheumatic drugs during pregnancy. Curr Opin Pharmacol. 2013; 13:470–475.
28. Petri M. Immunosuppressive drug use in pregnancy. Autoimmunity. 2003; 36:51-6.
29. Chambers CD, Johnson DL. Emerging data on the use of anti-tumor necrosis factor-alpha medications in pregnancy. Birth Defects Res a Clin Mol Teratol. 2012; 94, pp. 607-611.
30. Wakefield I, Stephens S, Foulkes R, Nesbitt A, Bourne T. The use of surrogate antibodies to evaluate the developmental and reproductive toxicity potential of an anti-TNF alpha PEGylated Fab’ monoclonal antibody. Toxicol Sci. 2011; 122, pp. 170-176.
31. Kurizky PS, Ferreira CC, Nogueira LS, Mota LM. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015; 90, pp. 367-375.
32. Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004; 99:2385-92.
33. Verstappen SM, King Y, Watson KD, Symmons DP, Hyrich KL, BSRBR; Control Centre Consortium. BSR Biologics Register Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011; 70:823–826.
Articole din ediţiile anterioare
Artrogripoza la un nou-născut prematur provenit din sarcină gemelară. Prezentare de caz
Artrogripoza congenitală multiplă (AMC) este caracterizată în literatura de specialitate de multiple contracturi articulare congenitale la nou-născ...
Care este comportamentul româncelor privind fumatul şi consumul de alcool în timpul sarcinii?
Deşi este arhicunoscut faptul că fumatul şi alcoolul au doar efecte nocive asupra sănătăţii, aceste obiceiuri afectează un procent important al pop...
Prognosticul materno-fetal al sarcinii complicate cu ciroză biliară secundară – review al literaturii de specialitate şi prezentare de caz
Managementul gravidei cu afectare hepatică reprezintă o adevărată provocare pentru echipa complexă formată din obstetrician, gastroenterolog, neona...
Nutriţia în sarcină
În sarcină, o dietă alimentară echilibrată în nutrienţi se asociază cu creşterea ratei de supravieţuire perinatale, creşterea greutăţii fetale la n...